Project Lead(s): Axel Guenther
Issue
Skin is the largest organ and forms a protective barrier against the external environment.
Following severe skin loss (>1sqm) as a result of burns, normal wound healing cannot reconstitute the barrier and multiple interventions are required for successful treatment.
The high cost and morbidity associated with current clinical procedures show the need for an innovative approach to developing skin substitutes.
Solution
This project aimed to further develop an approach for the scalable preparation of engineered skin substitutes.
A second-generation Skin Printer prototype was developed.
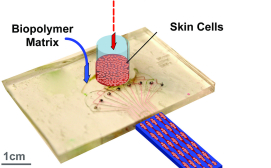
The Skin Printer is enabled by a microfluidic approach that converts cell-laden biopolymer solutions, upon interaction with cross-linking solutions, into cell-populated wound dressings that accurately reproduce the layered architecture of human skin.
The approach is based on a microfabricated printer cartridge that enables the scalable incorporation of skin microtissue regions within continuously produced hydrogel sheets.
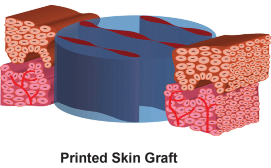
Printed skin grafts consist of multiple viable cell types, have a precisely controlled graft thickness, structure (e.g., epidermis and dermis) and biomolecular composition that are characteristic of human skin.
The Gen I Skin Printer is scalable to produce skin grafts up to 10 folds wider than the smaller grafts, equivalent up to 60mm-wide sheets. In vivo validation of cell-populated bilayered skin grafts was performed on 6mm-diameter incision wounds in immune-incompetent mice.
Outcome
Results from the project demonstrated that the Skin Printer allows the rapid formation of engineered skin substitutes.
However, the Gen I Skin Printer was found to have two limitations: it requires the manual manipulation of printed skin substitutes to a wound bed, and the applied method of producing free-floating biomaterial sheets limits the range of compatible biomaterials.
To overcome both limitations, the project designed and manufactured two units of a Gen II Skin Printer – a handheld skin printer that deposits adhesive skin substitutes directly onto the wound skin, removing the need for any manipulation of skin substitutes.
The printing method is compatible with a much wider range of clinically approved biomaterials, including collagen I and fibrinogen.
The Gen II Skin Printer has been protected with a provisional U.S. patent.
The team has continued the characterization of the Gen II Skin Printer with a small Medicine by Design bridge-funded project.
The technology was not deployed to the Children's Surgical Centre in Cambodia, as originally planned, because of unforeseen challenges in accessing cell biology infrastructure in that country.
Future project plans involve the continuation of in vivo experiments in small (mice) and large (pig) animal wound models, as well as the modification of the microfluidic bioprinter to meet cGMP requirements for use in humans.
The team is currently addressing the scalability of the skin printer cartridges through three successive MITACS internship grants being conducted in collaboration with SMT Americas (Burlington, Ontario) and z-microsystems (Austria).
The team has not yet decided whether to apply to Transition To Scale (TTS) Phase II funding.